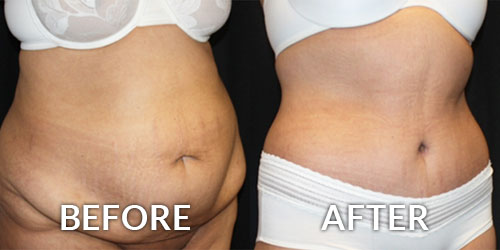
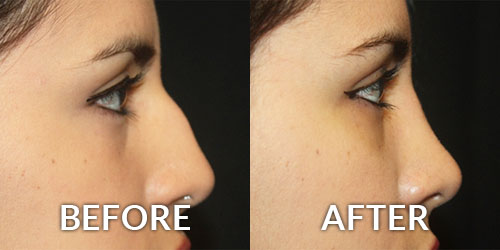
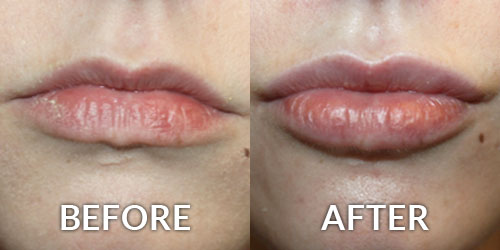
Allergan’s Juvederm Voluma XC is an injectable hyaluronic acid (HA) dermal filler for cheek augmentation to correct age-related volume deficit in the mid-face. The filler is a transparent, biodegradable, sterile, cross-linked hyaluronic acid hydrogel formulated to neutral pH in a physiological buffer.
If approved, Juvederm Voluma XC would be the first HA filler approved for correction of mid-face volume deficit. It’s designed to be a more robust filler with potentially longer longevity than those currently on the US market.
The FDA’s General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee voted unanimously in May to recommend approval of Juvederm Voluma XC. The FDA is expected to follow the panel’s recommendation, and, if it is approved, Allergan anticipates launching Juvederm Voluma XC in late 2013.
When Juvederm Voluma XC gets FDA approval for mid-face volume correction, it will be the first HA gel approved for this usage. The correction appears to last approximately two years.
Board Certified Plastic Surgeon Brian S. Glatt, MD, FACS, will keep you updated on the approval of this highly anticipated new product.