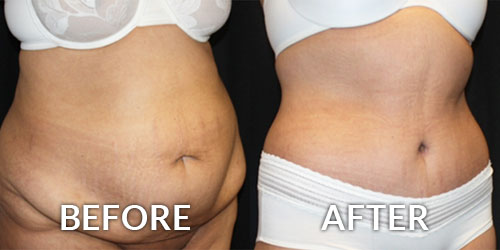
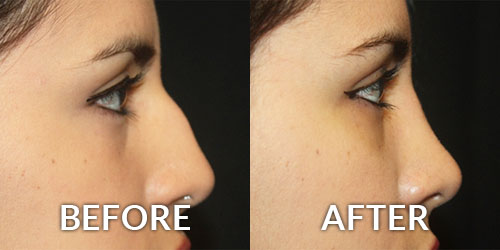
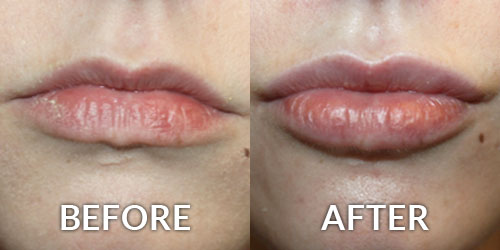
The United States Food and Drug Administration (FDA) has recently written to over 350 medical practices (23 in the tri-state area) about their medication purchases, which may include the purchase of unapproved Botox. The FDA stated these products may be “contaminated, counterfeit, ineffective, unsafe, and/or wrongly stored and transported” and “patients could be placed at risk”. Some physicians take advantage of “deals” by purchasing unapproved or illegal products from foreign suppliers. This is one way such low prices for these treatments can be offered. Board Certified Plastic Surgeon Brian S. Glatt, MD, FACS warns every patient “protect yourself from unscrupulous practitioners! If the deal seems to be too good to be true, then it probably is!” Patients receiving Botox should question where the product was purchased. Ask to see the vial, the box and the related medication guide, which is required by the FDA. No ethical physician should be put off by these requests.